Macitentan is the second nonselective endothelin receptor antagonist approved for PAH. It is a derivative of bosentan
Med Lett Drugs Ther. 2014 Feb 17;56(1436):15-6
The FDA has approved macitentan (ma" si ten' tan; Opsumit – Actelion), for oral treatment of pulmonary arterial hypertension (PAH). Macitentan is the second nonselective endothelin receptor antagonist approved for PAH. It is a derivative of bosentan (Tracleer), which is also manufactured by Actelion, and is scheduled to become available generically in 2015. Riociguat (Adempas), another new drug for this indication, will be reviewed in a future issue.
DRUGS FOR PAH — Current management of PAH usually includes warfarin (Coumadin, and others) and furosemide (Lasix, and generics). Oral drugs approved specifically for PAH include the phosphodiesterase 5 (PDE5) inhibitors sildenafil (Revatio, and generics) and tadalafil (Adcirca),bosentan, and ambrisentan (Letairis), a selective endothelin type A receptor antagonist. Patients with more advanced disease can be treated with multiple daily inhalations of a systemic prostacyclin such as iloprost (Ventavis) or treprostinil (Tyvaso), or continuous intravenous infusions of epoprostenol (Flolan, and generics) or treprostinil (Remodulin); treprostinil can also be given as a continuous subcutaneous infusion.
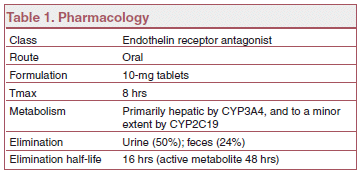
MECHANISM OF ACTION — Macitentan prevents the binding of endothelin (ET)-1 to both ETA and ETBreceptors. Macitentan has high affinity for ET receptors in human pulmonary arterial smooth muscle cells and may cause less liver toxicity than bosentan.
CLINICAL STUDY — Approval of macitentan was based on a clinical trial (SERAPHIN) in 742 patients randomized to placebo or to 3 mg or 10 mg of the active drug once daily. The composite primary endpoint was time from treatment initiation to the first occurence of death, atrial septostomy, lung transplantation, use of injectable prostanoids, or worsening of pulmonary hypertension. More than 60% of patients were on stable PAH therapy, primarily with oral PDE5 inhibitors.
After a median treatment duration of 115 weeks (the incidence of death was followed to 129 weeks), 31.4% of patients taking macitentan 10 mg/day experienced a primary endpoint event, compared to 46.4% of patients taking placebo (P<0 .001="" 6-minute="" a="" additional="" alone.="" an="" and="" as="" attributable="" authors="" beneficial="" but="" clinical="" defined="" did="" distance="" effect="" for="" in="" incidence="" lower="" macitentan="" morbidity="" mortality="" mostly="" need="" not="" of="" on="" p="" pah="" reduced="" reduction="" show="" significantly="" state="" symptoms="" that="" the="" to="" treatment.="" trial="" walk="" was="" which="" worsening="">
At 6 months, the secondary endpoint of 6-minute walk distance had increased by 7.4 meters and 12.5 meters in the 3-mg and 10-mg groups, respectively, and had decreased by 9.4 meters with placebo.
ADVERSE EFFECTS — Adverse events that occurred at a rate at least 3% higher with macitentan 10 mg than with placebo included headache, nasopharyngitis, bronchitis, and anemia. Decreases in hemoglobin and hematocrit have also occurred in patients taking other endothelin receptor antagonists. Macitentan is not recommended for patients with severe anemia.
Aminotransferase elevations, hepatotoxicity and liver failure have also occurred with other endothelin receptor antagonists. In the macitentan clinical trial, the incidence of elevated aminotransferases >3× the upper limit of normal (ULN) was similar with both doses of macitentan and with placebo, but the incidence of elevated aminotransferases >8x ULN was 2.1% with macitentan 10 mg and 0.4% with placebo.
A decrease in sperm count has occurred with other endothelin receptor antagonists and may also occur in men taking macitentan.
PREGNANCY — Macitentan is teratogenic in animals. It is contraindicated for use during pregnancy (category X) and is available for women only through a restricted access program. Pregnancy should be excluded before starting treatment and prevented during treatment and for one month afterwards.
DRUG INTERACTIONS — Strong CYP3A4 inducers such as rifampin can lower serum concentrations of macitentan, and strong CYP3A4 inhibitors such as ketoconazole and ritonavir can increase them. Concomitant use of macitentan with strong CYP3A4 inducers and inhibitors should be avoided.
DOSAGE, ADMINISTRATION, AND COST — The recommended dosage of macitentan is 10 mg once daily. Aminotransferases should be monitored before starting macitentan and as needed during treatment. The drug should be discontinued for clinically significant aminotransferase elevations or when elevations are accompanied by an increase in bilirubin >2x ULN or by symptoms of hepatotoxicity. Patients with pulmonary veno-occlusive disease should not take macitentan.
The cost of 1 month's treatment with macitentan is $6840.
CONCLUSION — Macitentan (Opsumit) can decrease morbidity in patients with pulmonary arterial hypertension, but the claim that it has been shown to decrease mortality is misleading